
Training is crucial for reducing the gap between nations
By Jan Huijs – HEART Consultancy, The Netherlands. jh@heartware.nl
Abstract
The international standards for sterile supply are embedded in Western societies; their implementation requires a strong economy. However due to many socio-economic limitations, transferring these standards – and the resulting advanced technology – to low-income countries is bound to fail. Manufactures are forced to comply with the standards and as a consequence appropriate sterilization equipment and related supplies for this market is hardly available anymore. A major problem is caused by the by the way the requirements for validation of processes have been defined. The current situation related to autoclaves (sterilizers using steam as sterilizing agent) in target health facilities was analyzed; followed by research and development and resulted in prototypes that eventually should mature to an adequate autoclave for the rest of us.
1. Situation analysis of sterilization equipment in target locations
Socio-economic context
Many health facilities have to operate under harsh conditions with poor and/or limited water supply; poor/limited or even no electricity supply; and often long distances over a very poor road network. Well trained staff is extremely scarce and companies available may be very expensive, especially for a remote hospital that may be hundreds to a thousand of kilometers away from the capital, making only the trip of the technician to come to the site already a financial challenge for the hospital.
Mind the gap

The difference between the average spending on health care per person of a population in high income and low income countries is huge. For example in the United States this spending may be approx USD 8200 per person pear year, whereas in Malawi it is as little as approx USD 74 [2]. A difference of a factor 110! It is obvious that technology developed in high income society, assumes the infrastructure of such society: The technology and the resulting products are embedded in their society. And usually requires the infrastructure and related financial resources to keep them operational. The incredible big gap of financial resources and all its implications makes it obvious that advanced equipment developed to be used in the industrialized world is bound to fail when taken to a society where only a fraction of the financial resources are available.
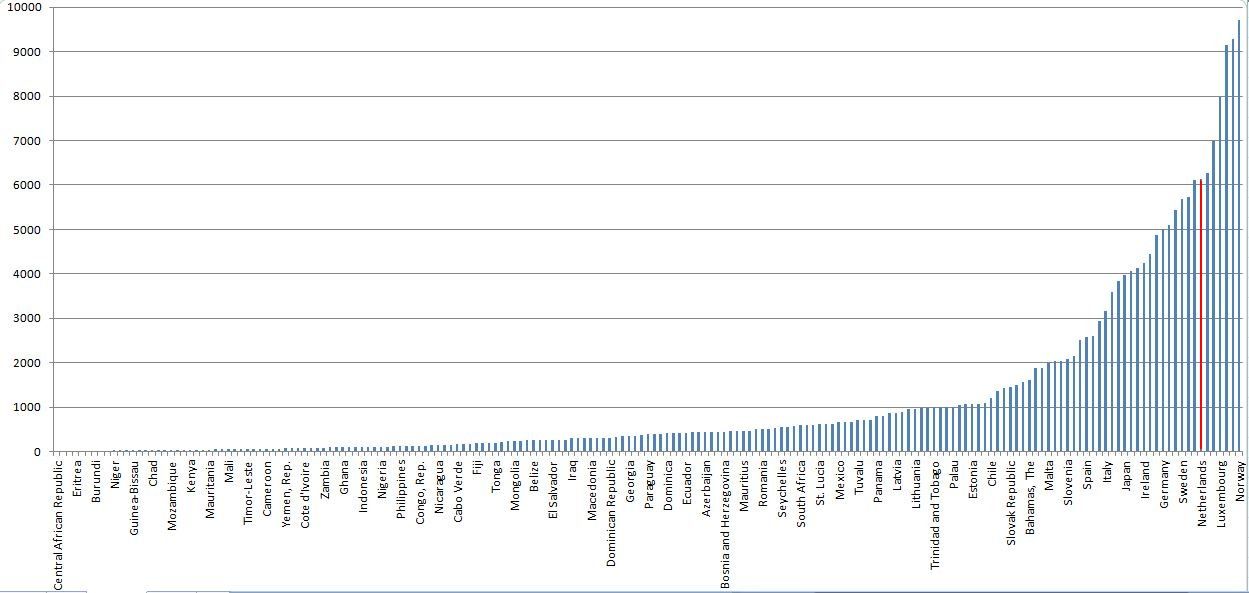
WHO: 2013; Country’s per capita spending on healthcare
Why do mobile phones thrive and autoclaves die?

2009-11-14: New autoclave donated by the French Embassy; never used. Bangui, CAR. No further technical support nor adequate training was provided. Reason of malfunctioning: lack of training; no compressor for pneumatic valves was supplied.
Africa is undergoing a communications revolution. Mobile phones have become accessible to major parts of the populations. Mobile phones are very high tech devices; which in spite of that are a huge success in also remote African settings. Whereas advanced autoclaves are found broken down and not used in many places. A major reason here are the economy of scale and the cost per item. Mobile phones are used by the tens of thousands. For such quantities it is economically viable to set up an infrastructure to have the network and the equipment operational. Moreover the price is relatively low and thus are affordable for most individuals. In addition the telephone has a tremendous positive impact on virtually everybody’s life. Autoclaves however are only used in health facilities; thus quantities are very low; while the diversity of makes and models is huge. Of some make/model there may be only a single unit in the country. This makes it for a manufacturer/supplier economically not viable to set up a service network. It simply is too expensive. Thus, there is no or virtually no support for the ever more complex equipment, resulting in many broken down machines. In addition the requirements for utilities such as water and electricity has become more stringent making the equipment even more vulnerable for breakdowns.
Standards and legislation
As for may other fields of industry and services, a wide range of standards related sterile supply have been compiled. Main objectives are to assure safety and health of users and patients, to provide a minimum level of quality and to facilitate international trade. In Europe the standards are issued and managed by CEN (Committée Européenne de Normalisation) whereas ISO (International Standards Organization) compiles standards that are to be worldwide. Compliance with standards is voluntary; however meeting the requirements of standards can be used to demonstrate compliance with the relevant legislation; in the European Union this is the Medical Device Directive. In the context of the Medical Device Directive sterilizers are medical devices and thus sterilizers must comply with the directive. In order to complement the European legislation, governments may have additional national laws. Due to several (mostly economical) reasons, the target countries in the developing world however are not or cannot be members in these committees. Sub-Saharan Africa has not a single member. Therefore the specific problems related to these countries are not considered in the formulation of the standards. The content of the standards is based on the assumption that they are implemented in the member countries. The standards thus are embedded in the richer, Western societies in which the often totally different conditions of the non-member countries are not considered.
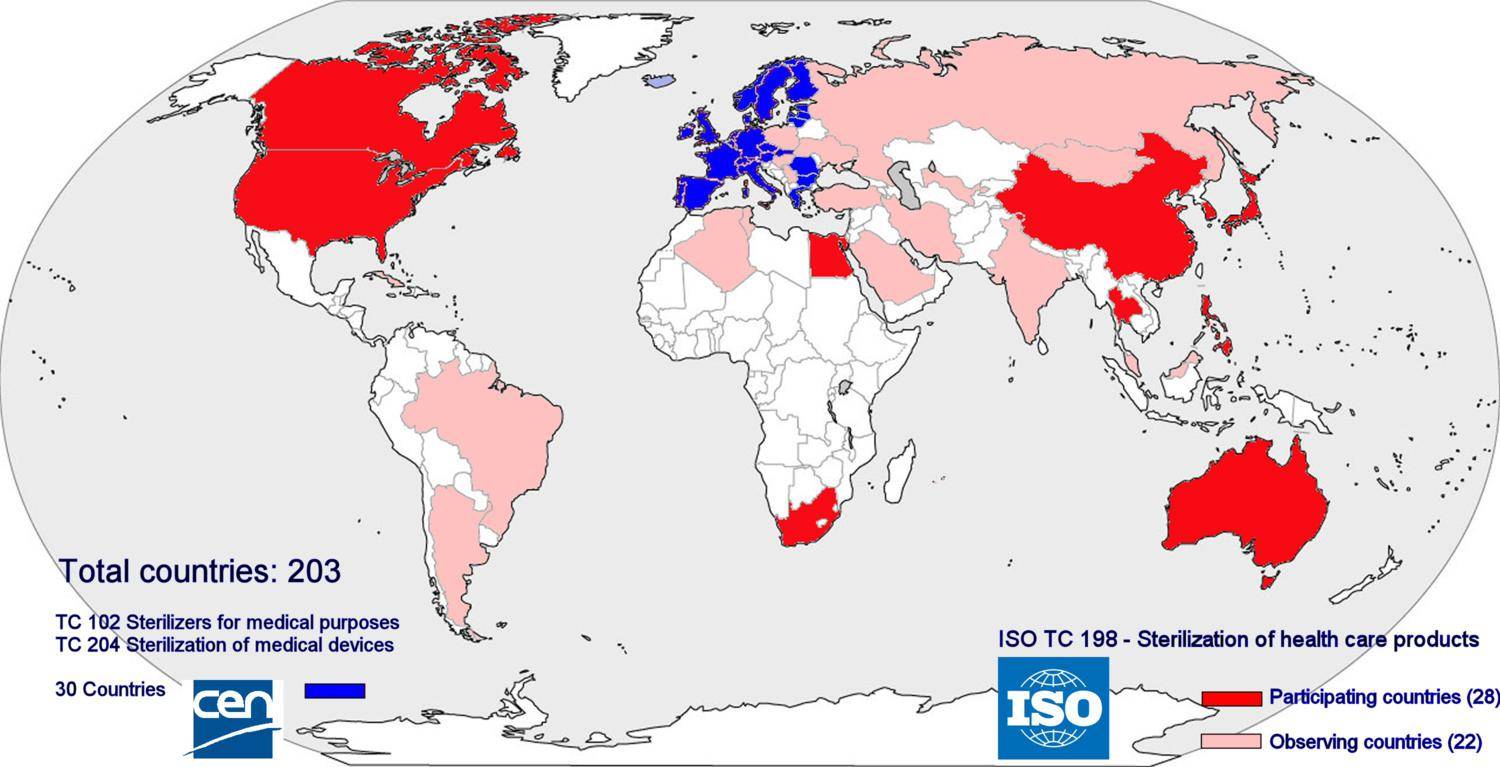 Membership of ISO and CEN. No low income countries are member of the Technical Committees (TC) related to sterilization in the standards organizations and thus have no influence on the standards that are formulated
Membership of ISO and CEN. No low income countries are member of the Technical Committees (TC) related to sterilization in the standards organizations and thus have no influence on the standards that are formulatedValidation excludes manual control. Some observations
It is a requirement of the standards on sterilizers that of any autoclave its processes are validated. This implies that there is to be documented evidence that the autoclave renders sterile products and that the process is reproducible. In this context human actions are not considered reproducible and thus also manual control of a machine is not reproducible. In the sequencing of a process, wherever possible, the human factor must be excluded. However, when I, together with many other people board a bus, I put my life in the hands of the bus driver. His actions cannot be validated. We rely on him. The responsibility of the bus driver is not less then a person operating a sterilizer. But still busses are manually driven. And is accepted everywhere. Why then not, at least in locations where advanced technology is not feasible, accepting manual control for sterilizers by well trained operators using reliable autoclaves that result in adequate sterile goods when operated well?
State of the art of sterilization equipment: fully automatic autoclaves. Often a headache for remote hospitals
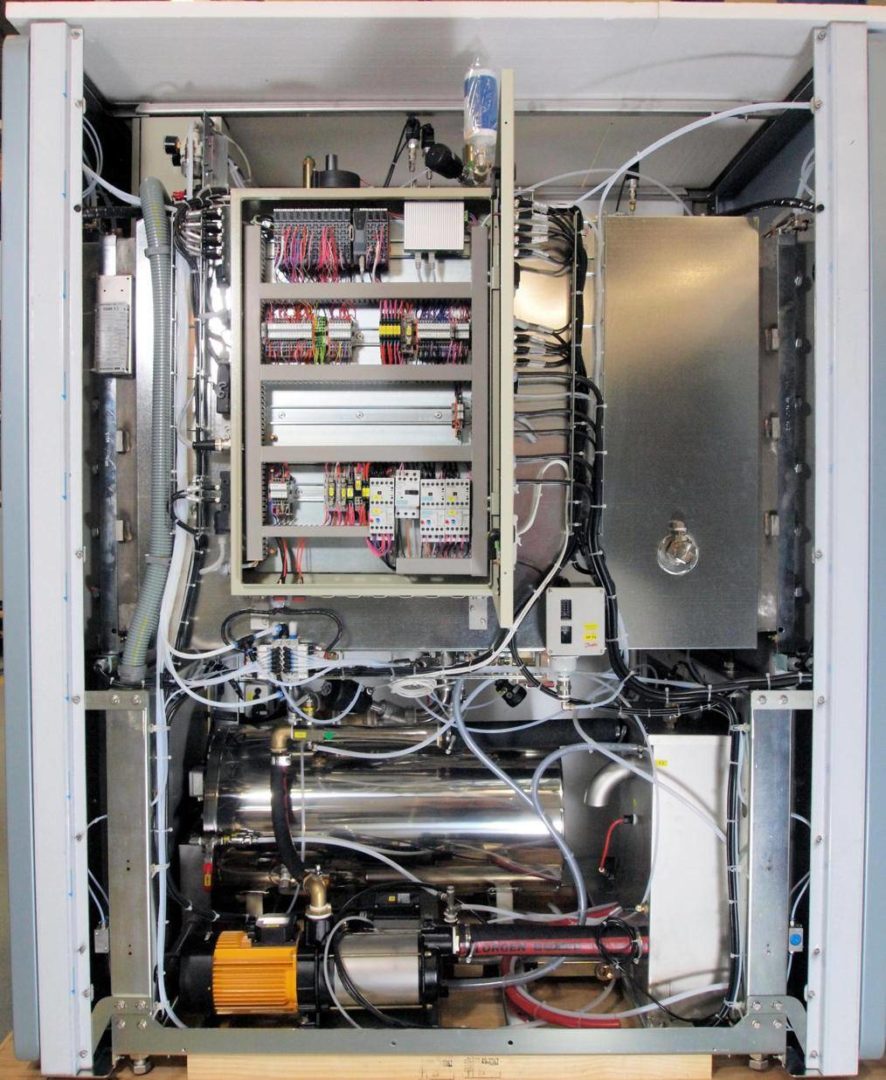
2014-03-25: A look Inside of an advanced computer controlled sterilizer.
Based on the concept that human actions are not reproducible and thus that only automatic autoclaves can provide validated processes, it became a requirement that processes for autoclaves are automatic. With the current technology this implies that autoclaves are microprocessor controlled. This results in equipment with a large quantity of high-tech components with a complex control mechanism. An example: In modern standard autoclaves the operation of a valve for steam supply requires a multitude of components: the microprocessor with its software; a relay; an electric valve controlling pressurized air; and the final pneumatic valve that controls the actual supply of steam. Thus there is a chain of multiple components for a single function with each component increasing the machine’s complexity and increasing the risk of breakdowns.
2. Focus on manually operated sterilizers

2011-10-04 Bungudu Hospital, Nigeria. In many situations a manual sterilizer is more appropriate and reliable than the latest high-tech autoclave that meets all standards
In many health facilities in developing countries, the use of complex automatic sterilizers as required by the current international standards are not feasible. For many facilities they drain the already very tight hospital budget in many cases hospitals have to resort to very basic, unsafe poor performing manual sterilizers.
Accepting manual controlled sterilizers
For this market, more robust, well performing autoclaves are required, based on manual control and without the need of complex electronic control and related components. By allowing manual control of a sterilizer a drastic reduction of complexity and number of components can be accomplished and resulting in a more reliable essential sterile supply in the target health facilities.
Approval of standards organizations and WHO/health authorities
Allowing manual control in the context of validation would require approval of the authorities related to the publication of the standards. It is therefore is recommended to open a debate on this issue with the relevant organizations such as CEN/ISO, WHO and possible other stakeholders. Acceptance of manual control by such respected organizations will be crucial for the commercial success of any approved sterilizer suitable for the target market.
Large potential market
The target market for the sterilizer would at least be all low income countries. However large regions of middle income countries, the socio economic situation also does not qualify for a reliable operating of advanced equipment. Thus the market for such sterilizers would include a major part of the worlds’ countries; which are home to a major part of the world population!
Essential requirements for equipment
For any piece of equipment, in the context of this article a sterilizer, to provide its intended function throughout its lifespan, it should meet the following requirements
- It should do its job: For an autoclave in our contex this implies that it sterilizes materials as required in the target health facilities e.g. common remote general district hospital or health centre in the developing world.
- The equipment meets the local socio-economic setting where it is to be used; in other words, the machine should be able to run during its estimated lifespan against reasonable cost in the target location. This implies that its operation, maintenance and repairs should be compatible with the annual budget for a remote moderate hospital.
Performance requirements specific to sterilizers
For a sterilizer two fundamental performance requirements can be identified
- Processed goods must be sterile according to the requirements for an item to be labeled sterile as formulated in the Standard EN556-1. The sterilizer thus must render products sterile inside their packaging.
- Processed goods must be dry as specified in standard EN 285
Characteristics of goods to be sterilized in a typical district hospital
In any health facility sterile materials are used; a part of them may be single use/disposable; a large number of items are reusable and must be cleaned, packed and resterilized after each use. In a typical hospital reusable sterile materials are used in the operating theatre, delivery room and treatment rooms. These items are to be reprocessed in the Central sterilization department of the hospital:
- Wrapped surgical instruments. They may be in drums or containers. Some surgical instruments may have lumen of limited length such as suction tubes, cannulas. Instruments for MIS (Minimally Invasive Surgery) with long narrow lumen are in general not used. Such instruments require a more advanced autoclave with a pre-vacuum process.
- Wrapped textile goods/porous loads, such as gowns, drapes, swabs etc.
- A combination of these
Process profile
For a steam sterilizer to render sterile products for use in a hospital environment, the process has the following phases
- Air-removal. Air acts as an insulator for heat transfer by steam. Therefore in order for the steam to be in touch with all exposed objects, all air needs to be removed from the chamber and the load
- Sterilization. The actual sterilization phase during which all micro-organisms are killed. For saturated steam the standard minimum parameters are: 134ºC for a minimum of 3 minutes; or 121ºC for a minimum of 15 minutes
- Drying. Moisture is a breeding ground for micro-organisms. That is why at the end of a sterilization cycle the load should be dry. This is accomplished by creating a vacuum, causing any residual moisture to evaporate
- Air-admission. At the end of the drying cycle, the chamber pressure is below atmospheric, thus the lid cannot be opened. Only after equalizing the pressure in the chamber to atmospheric, the lid can be opened. For preventing recontamination of the load, the air entering the chamber should pass a high quality bacteria filter
Ensuring adequate performance of manually operated sterilizers: research
Given the socio-economic context of the target countries, one of the major requirements would be that it should be possible to use manually-operated sterilizers. Automatic control can be an option, but manual control should remain possible.
It therefore was essential to get scientific evidence that autoclaves that are manually operated can actually result in sterile goods that meet the set standards for sterility and dryness. That is why already in 1999, a research programme was implemented, in collaboration of the medical engineering department of a college on technology (Hogeschool Enschede, (NL) and the Dutch Institute for Public Health and the Environment RIVM [1]. The research took place in one of the laboratories of the RIVM. All procedures regarding the performance testing of the sterilizer were done based on the prescribed performance test methods as formulated in the respective standard for such studies (EN285). During the study two types of manually operated sterilizers, at the time common in district hospitals in developing countries were tested. Its most important conclusions.
- Sterilization achieved. The requirements for sterilization of wrapped instrument and porous loads can be met in these sterilizers, provided that adequate air-removal is achieved, by adhering to a process that was the result of the research which includes steam flushing and (3) above-atmospheric steam pulses.
- Dryness achieved. Adequate drying is feasible by using steam condensation. Therefore there is no need of an electric waterring pump, the technology that is widely used in main stream sterilizers. Using a condenser, there is no need of electricity, there no moving parts and only limited or no water is required.
- Waterring pump eliminated. With the performing of the steam penetration by above atmospheric steam pulses and creating an effective vacuum by a steam condenser, the need for an electric vacuum pump is eliminated. The use of the steam condensor reduces the vulnerability of the autoclave and reduces water consumption of the autoclave tremendously.
- Manual control is complex; more simple operation is needed. For a successful sterilization process, the operation of the 4 valves at the correct moment and correct sequence is critical. It makes operating the autoclave rather confusing and prone to operator errors. It was suggested to do further research in order to reduce the complexity for the operator.
Optimizing manual process control
Objective for this part of the research was to drastically reduce operator errors by reducing the number of controls/valves to be operated. In the conventional manually operated autoclaves on the market, each individual valve needs to be operated separately. Valves are at several locations on the machine. It thus requires operating multiple valves; usually 4 valves in the correct sequence and the correct moment. In several situations 2 valves need to be operated simultaneously. This method of operation requires a strict protocol and very much dedication of the staff. It thus is prone for operating mistakes. Therefore a solution was searched for by single-knob mechanical control of all valves. The research was done in collaboration with the Technical University in Eindhoven, The Netherlands in the period of 1999 until 2004. Several mechanisms were tested. However control by a camshaft system with multiple camdisks proved to be the most efficient, flexible, user friendly and cost-effective solution. It is a technology that has been used in autoclaves in the 1960’s and 70’s already but was abandoned when electronic control systems took over.
Right: The camshaft controller with its 4 valves. After a stepping the handle through its distinct 12 positions, the full sterilization cycle is completed.On the right hand side the air-cooled condenser for creating the vacuum for dryingRight: The camshaft controller with its 4 valves. After a stepping the handle through its distinct 12 positions, the full sterilization cycle is completed.
On the right hand side the air-cooled condenser for creating the vacuum for drying
 |
 |
| Second generation prototype of the autoclave as installed in a district hospital in Eikwe, Ghana. 2008-06-09 | The camshaft controller with its 4 valves. After a stepping the handle through its distinct 12 positions, the full sterilization cycle is completed. On the right hand side the air-cooled condenser for creating the vacuum for drying. 2007-11-16 |
The camshaft controller has a number of advantages:
- Very simple operation: a single knob for operating all valves, ensuring the right sequence of operation of valves which is critical for a correct process; thus chances for wrong operation is drastically reduced. Each step in the sterilization process is a distinct step of the control knob. Each disk will put each valve at its correct open or closed position. A full rotation in 12 steps results in a full, validated process for general hospital loads
- Valves can be operated mechanically by cams; no need for electric/pneumatic valves; reducing number of components considerably. The control system can work fully independently of electricity.
- Very sturdy, robust design; built for a lifetime
- Repairs can be done by a well trained plumber
- Process profiles can be changed by changing the shape of the disks
- The concept can be used for single chamber as well as double-chamber autoclaves
- The concept of the system allows automation by adding the required sensing and control components. An upgrade kit could be made available, including sensors, timers and a motor drive for the controller. As it would provide fully automatic control and would open the possibility to have this upgraded model pass the requirements for the current standards. And in case of a breakdown of the electronic control part of the system, it allows to resort to full manual control.
3. Prototyping and scaling up
5 prototypes of several generations were built and field tested in 3 countries in Africa (Ghana, Central African Republic, Zimbabwe). They are jacketed sterilizers, equipped with the camdisk controller and an air-cooled condenser as vacuum system. The first prototype was installed in 2005 has been running for 8 years without repair on the control system. The next phase is to find manufacturers in order to scale up production and finally come to an adequate commercially available autoclave that the health sector in many countries is waiting for.
4. Discussion
The requirements of current standards have resulted in advanced medical equipment requiring the infrastructure and budget of an industrialized nation. There however is a dire need of equipment that meets the harsh local socio-economic background of many developing countries. A major cause of this situation is the lack of adequate standards and guidelines which all stakeholders involved in health care in the developing world are facing. It is crucial that this huge gap in the quest for quality, that standards organizations are committed themselves to, will be filled in. A gap that now causes sterilizers that meet all standards, and that finally (may) reach their destination in a remote hospital, may never run a single cycle. Through research it has been demonstrated that adequate sterilization can be performed in manually controlled sterilizers. Also by eliminating the electric waterring pump the complexity and water consumption can be reduced tremendously. A manually operated camshaft control system reduces operator errors considerably and thus improves reliability of the process. An add-on kit could make the unit fully automatic, opening the way to meet the requirements of the standards and still have the possibility to resort to manual control. The author is in the process of forming a working group that will address the issue of an adequate sterilizer for the rest of us.
5. Acknowledgements
I herewith thank all those dedicated people involved during the years of research, prototype building and field testing!
6. References
[1] B. Muis, ACP de Bruijn, A.W. Van Drongelen, J.F.M.M. Huijs. “Optimalization of the process for manually operated jacketed steam sterilizers”. Zentral Sterilization/Central Service 2002-10 (6), page 373-384
[2] WHO Department of Health Statistics and Informatics (May 15, 2013) “World Health Statistics 2013”. WHO
First published and presented during the congress on Appropriate Health Technology for Low Resource Settings 2014 – (AHT 2014), London, organized by the Institute of Engineering and Technology, UK. www.theiet.org/aht2014
 Jan Huijs is the owner of HEART Consultancy, based in the Netherlands. After working as a medical equipment engineer in Ghana, Africa for 7 years in the 1980’s he started HEART Consultancy, a consulting agency focusing on sterilization of medical supplies and asset management for health facilities, focusing on the health services in the developing world. He presents training for users as well as technicians on sterilization of medical supplies in mainly developing countries since 1997. He is author of the book “Sterilization of medical supplies by steam”, which has been translated into 8 languages. He has been doing research on sterilization of medical supplies in remote areas.
Jan Huijs is the owner of HEART Consultancy, based in the Netherlands. After working as a medical equipment engineer in Ghana, Africa for 7 years in the 1980’s he started HEART Consultancy, a consulting agency focusing on sterilization of medical supplies and asset management for health facilities, focusing on the health services in the developing world. He presents training for users as well as technicians on sterilization of medical supplies in mainly developing countries since 1997. He is author of the book “Sterilization of medical supplies by steam”, which has been translated into 8 languages. He has been doing research on sterilization of medical supplies in remote areas.
Since 2014 Jan is honorary member of SVN; Sterilisatie Vereniging Nederland, (Dutch Sterilization Association).
